The effect of alkali and earth alkali metal oxides on kinetic of wollastonite growth in melt
Izvestia Academia Of Science USSR
Inorganic Materials
1985, v.21, N 4, p. 639- 641. (
offprint rus.)
The processes of crystallization in silicate melts play an important role in various technological processes, such as stone casting, the work of metallurgical slags, the production of glass-crystal materials [1-9].
The purpose of this work is to study the effect of additives of alkaline (Li, Na, K) and alkaline-earth (Mg, Sr, Ba) metals oxides on the growth rate of crystals wollastonite CaSiO3 in the melt. Wollastonite was chosen as an object of investigation insofar as it has a relatively simple well-studied structure [10], and is also the main crystallizing phase in vitreous slags of phosphorus production.
Temperature dependences of the growth rates of wollastonite crystals were obtained by means of a high-temperature microscope [11]. Features of the crystallization of melts required the development of new methodological techniques. The upgraded specimen heater-holder, made in the form of a strip of Pt + 30% Rh alloy with a narrow (0.1 mm) slit (2 mm long) into which a drop of melt was placed, allowed to increase the range of measured crystal growth rates and phase transformations by an order of magnitude.
The high rate of evaporation of alkaline oxides at temperatures above the liquidus temperature caused a change in the composition of the melt during the investigation. Continuous monitoring of the composition of the sample in magnitude liquidus temperature [12, 13] made it possible to study the kinetics of crystal growth in melts of different compositions on a single sample.
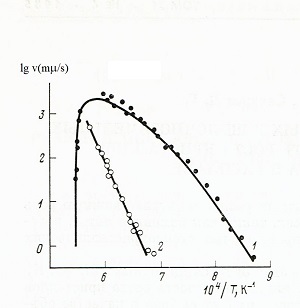
Fig. 1 |
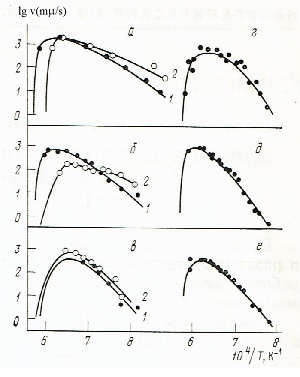
Fig. 2 |
|
Fig. 1 Temperature
dependences of the linear growth rates of crystals and phase transitions of
wollastonite (1-growth of crystals of melt, 2-polymorphic conversion
of wollastonite to pseudowollastonite).
|
|
Fig. 1 presents data on temperature dependences the growth rates of wollastonite crystals from the stoichiometric melt. There are two growths of its modifications: up to 1300 K - low-temperature, above this temperature, its high-temperature modification pseudowollastonite. During the transition of wollastonite to pseudo-wollastonite, the movement of the boundary between two modifications was visually recorded, which helped determine the temperature dependence of the polymorphic transformation.
Data on temperature dependences of linear growth rates crystals in the wollastonite melts by the addition of oxides of alkali and alkaline earth metals are shown in Fig. 2. The amount of additives was chosen so that the composition of the melt remained in the field of crystallization of wollastonite. The liquidus temperatures of the investigated compositions were not lower than 1720 K.
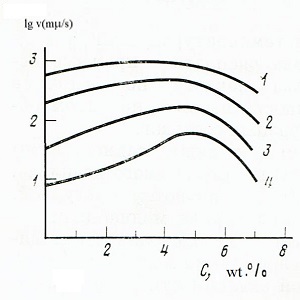
|
| Fig. 3 Dependences of the growth rate of wollastonite crystals on the composition of the melt at constant supercooling (1 - 300, 2 - 400, 3 - 500, 4 - 600 K) |
Additions of lithium oxide caused a sharp increase in the growth rate crystals. the dependence of the crystal growth rate on the composition for identical supercoolings ΔT = To-T have a maximum in the range 4-5.5 wt. % Li2O, which corresponds to the compound with the gross formula Li2CaSixO3x + 1, where x is equal to 6-8 (Fig. 3). X-ray patterns of crystallized melts revealed two new crystalline modifications. High-temperature is an analogue of pseudowollastonite. Its X-ray diffraction shows the splitting of peaks characteristic of pseudowollastonite, which indicates the presence of a superstructure. X-ray patterns of low-temperature modification and wollastonite do not have common peaks.
The growth rate of crystals from a melt containing up to 3 wt. % Na20 is less than from a melt of stoichiometric composition. Increase the Na2O content slightly increases the crystal growth rate at low temperatures due to the formation of a metastable crystalline phase into which sodium enters.
Additions of potassium oxide to 10% weight. % slowed the growth rate of the crystals. The increase in pseudowollastonite from rafts, containing more than 3 wt. % of potassium or sodium oxide, vigorous gas evolution was observed in the last stages of crystallization, which is explained by the melt enrichment before the front of crystallization with sodium or potassium oxide and "boiling" it when the oxide concentration exceeds the critical value for this temperature.
Small additions of alkaline earth oxides equally affect the growth rate of wollastonite with a change in temperature. They more or less sharply reduce the rate of its growth at a given temperature as compared with the growth of the stoichiometric composition on the melt. The experimental data were approximated by a semi-empirical equation [14]
v = k (Δ/T)c×exp (-E/ RT) (1)
where k, c, E are empirical coefficients. The table presents the results of mathematical processing of experimental data by the least squares method on a BESM-6 computer. The quantity c entering into equation (1) is determined by the mechanism of the transition of structural elements from the melt to the crystal and depends on the structure of the melt and crystal [14].
Additions of lithium oxide lead to a sharp drop in the coefficient c, which, apparently, is associated with the growth of new crystalline phases. A sharp increase in the coefficient c in the case of small additions of potassium oxide is probably due to a substantial change in the structure of the melt near the growing wollastonite crystal, caused by the accumulation of potassium ions that do not enter the crystal structure.
| Addition | Weight, % | c | ln(k), (μm/s) | E, (kJ/mol) | η2 | T, K |
| - | - | 2.69 | 39.5 | 367.5 | 0.988 | 1830 |
| Li2O | 5 | 1.12 | 27.7 | 234.5 | 0.991 | 1780 |
| Li20 | 7 | 1.44 | 26.9 | 215.5 | 0.985 | 1735 |
| Nà20 | 5 | 1.7 | 34 | 314 | 0.979 | 1750 |
| Na20 | 10 | 2.75 | 30.7 | 258 | 0.9 | 1720 |
| K20 | 5 | 5.82 | 69 | 650 | 0.994 | 1750 |
| K20 | 10 | 3.13 | 52.6 | 507 | 0.999 | 1725 |
| MgO | 3 | 3.54 | 56.2 | 548 | 0.87 | 1750 |
| SrO | 13 | 2.03 | 52.8 | 550 | 0.984 | 1750 |
| Ba0 | 11 | 2.51 | 51.2 | 522 | 0.989 | 1740 |
Thus, the effect of small additions of alkali and alkaline-earth metal oxides on the crystal growth rate from the melt can be manifested not only by lowering the liquidus temperature and decreasing the driving force of crystallization, but also by modifying the structure of the melt and the crystal. An increase in the growth rate occurs if the metal ion is capable of modifying the structure of the main phase in such a way that it approximates the structure of the melt. If the ion that strongly modifies the structure of the melt does not enter the lattice of the crystal, it sharply slows down the growth rate.
CONCLUSIONS
It has been established that small additions of alkali alkaline
earth metal onsides in the decomposition can both increase. and
to reduce the growth rate of the crystals of wollastonite.
Literature
1. Zchimer E. The velocity of crystallization in soda lime silica glasses. J. Soc. Glass. Technology, 1 929, v. 13, M. 49, p. 76.
2. Kumm KA, Scholze N. Die Kristallisationsgeschwindigkeit von Shlackenschmelzen in System CaO A12O3 SiO2. Tonind-Ztg, 19169., v. 93, v.2 N 9; p. 332, 10, p. 360.
3. Sheludyanov L.N., Dyshlova T. A. About interrelations between the composition, structure, and crystallization ability of the melts of the CaO-Al2O3-Khabarshysy West system, Vol. 35, No. 7, 1976, p. 11.
4. Shkolnilov E.V. , K Kinetics of Crystal Growth in Glasses M20 • 2SiO2 (M - Li, Na, K) .- Physics and Chemistry of Glass, Vol. 6, 2. 1980, p. 153.
5. Kirkpatrick R. J. Kinetic of crystal growth in the system CaMgSi2O6-CaAl2SiO6. - Amer. J. Sci., 1974, v. 274, 3, p. 215.
6. Siroko N.P. Study of the crystallization process of glasses of the gehlenite - wollastonite system: Dis. Sci. Art. Cand. Tech. sciences. l .: IXS USSR Academy of Sciences, 1971.
6. Siroko N.P. Study of the crystallization process of glasses of the gehlenite - wollastonite system: Dis. for the competition uch. Art. Cand. tech. sciences. L .: ISC USSR Academy of Sciences, 1971.
7. Rumyantsev P.F., Sakharov L.G. The nucleation and growth of crystals in a pseudo-binary system gehlenite - a two-barrel spiral - Izv. AN SSSR. Inorgan. Materials, 1981, v. 17, N'2 3, p. 466.
8. Rumyantsev PF, Sakharov LG Crystallization of glass-forming melts of the anorthite-gelenite system .- Physics and Chemistry of Glass, 1981. Vol. 7. N2 2, p. 159.
9. Rumyantsev P.F., Sakharov L.G. Crystallization in pseudobinary systems anorthite-gehlenite and gelenite-dicalcium silicate .- International. Conf. on the crystals growth. T. 3, Moscow: Publications of VINITI, 1980, c. 274.
10. Minerals. Moscow: Science, 1981, vol. 3, no. 2.
11. H. Leonov A.I., Rumyantsev P.F. High-temperature microscope. Moscow: Published by. VDNKh.
12. Belyannin DS, Lapin VV, Toropov NA Physical and chemical systems of silicate technology. Moscow: Promstroyizdat, 1954.
13. West A. R. Phase Equilibrium in the system Li2O-CaO-Si02.- J. Amer. Ceram. Soc., 1978, v. 61, N 3-4, p. 152.
14. Rumyantsev P.F., Sakharov L.G. Influence of the structure of metal and slag melts on the kinetics of the surface reaction of crystal growth. - In.: Improving the quality, reliability and durability of products from structural, heat-resistant, powder and tool steels and alloys. L.: Publ.. LHNTP, 1983, c. 74.
Mar. 23, 2018; 12:10 EST