Types of molecular structures on the crystal surface.
A molecular structure of the crystal surface is defined the possible mechanisms of its growth. The rough surface produce continues mechanism, absolute smooth flat surface corresponds to two dimensional nucleation, a presence of dislocation leads to the spiral mechanism.
The model of thermally activated crystal growth as described in the article leaves aside the very important feature of crystal growth phenomena namely the influence of surface energy. In this model all positions on the rough crystal surface are presumed equally able for accepting and emitting molecules. In fact it is a true but only when we are speaking in average. Molecules in valleys are less probable to emit from crystal than average those on tops of "hills" are more probable to. In average all positions are equal for absolute rough surface.
A difference in levels of free energy between molecule situated at the surface of the crystal and belonged to crystalline phase from one side and similar molecule that belonged to amorphous phase on other side is defined not only difference between thermodynamical potentials of crystalline and amorphous phases but also by addition of component that reflect on change surface of crystal in case of such molecule transformation as is shown in formula:
| ΔG = ΔμVm+ σΔSm | (1), |
where Δμ - change of chemical potential in crystalline and amorphous phase, σ- surface energy of border between crystal and amorphous phase, Vm - volume of molecule, ΔSm - change of surface of crystal as result of transformation of molecule. The change of surface if molecule come out of crystal can be expressed from part of this molecule on surface that is in direct contact with neighbors in crystalline phase:
| ΔSm= Sm - 2Scr | (2), |
where Sm - surface of whole molecule, Scr - surface of the part of molecule in direct contact with other molecules in crystalline phase.
Should all molecules on the crystal surface have exact half of their outer area belonged to crystal there would be no reason to incorporate surface energy into formulas for crystal growth. In reality it is geometrical impossibility. The general solution in form of simple formulas for growth rate of crystal surface that incorporated surface energy seems like matter of impossibility at least in form of mathematical theorem. There main problem is that one of the parameters of solution is the roughness of the growing crystal surface but there are no independent principles to help to define it any other way than direct computer simulation.
A following figure shows a typical snapshot of positions of molecules on the crystal surface produced by computer simulation:
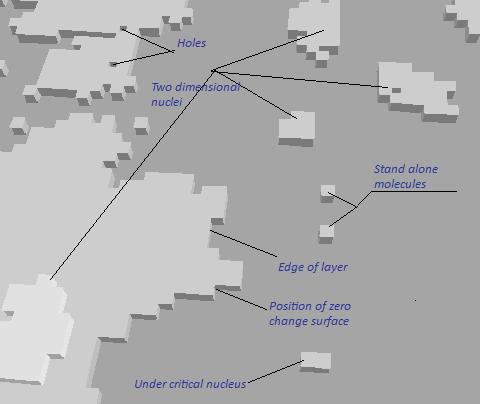
Thermodynamically the most advantageous configuration of molecules is the flat, completely filled layer. After one layer is completely filled to start completion of the next layer a formation of island called two dimensional nucleus has to happen. On the larger size analogous to formation of activation complex situation has to happen. The same as for activation complex in chemical kinetics a change of free energy to form two dimensional nucleus has to be positive. It is thermodynamically prohibited in sense of direction of system transformation on the macro scale. The trick is that two dimensional nucleus is small enough to appear as result of random fluctuation when during chaotic elementary moved of molecules on a smooth surface in and out of crystalline state favorable result will occasionally happen by stroke of luck. A separate article is devoted to detail discussion this mechanism of two-dimensional nuclei growth.
To take around fluctuation mechanism one can postulate an existence of permanent edge on the surface of growing crystal these are called spiral (screw) dislocation. The idea is that such dislocation can be permanent substitute to random formation of overcritical nuclei. If some deviation of ideal structure during the growth is happen like isomorphic substitution of atoms or forming of hole inside there could be permanent activation centers on the smooth surface substituting two dimensional surface. More details about dislocation induced crystal growth can be found in separate article.
The main premise of two-dimensional and dislocation mechanisms of crystal growth is presence of layer by layer growth. It means that as a rule until one layer is completely filled next one is not begins to growth. As obviously can be seen from the picture above this assumption in most cases is more exception than a rule. The broad attention to layer by layer mechanism is due to most practical importance for the industrial production of big bulk crystals when technological parameters of process are specially set to reach as perfect crystals as possible. But actually a number of important applications in industry and science when layer by layer model in its pure form is not realized are quite enormous.
In reality crystals are formed in variety of shapes from nice snowflakes, dendrites, spheres, needles and many others. It is makes difficult even to find adequate nicknames for all of them. There are obvious simplification of the very complex situation when model has omitted a phenomena of transportation of molecules to surface of crystal from feeding neighborhood and exhaust of heat and alien molecules away from from crystal surface.
Sep. 28, 2017; 11:32 EST